1. INTRODUCTION
Diabetes is the main cause of mortality, morbidity, disability and healthcare expenditure worldwide [1]. Since 1980, the burden of diabetes in developing countries has increased significantly faster than in developed countries [2]. The prevalence of diabetes and pre-diabetes in Vietnam between 2010 and 2015 has doubled over 5 years, from 2.6% to 4.1% and 1.5% to 3.5%, respectively [3]. In 2016, a study using HbAlc to diagnose diabetes found the prevalence of diabetes and pre-diabetes in individuals with age over 30 years in Ho Chi Minh city were 12.3% and 40.1%, respectively [4]. Diabetes has become a public health burden in Vietnam as a result of several socioeconomic changes and culturaltransitions [5].
Living with diabetes could be exhausting, tough and overwhelming struggle, which emphasized the need of a comprehensive diabetes care program not only focus on clinical outcomes but also explore the potential behavioral and psychological issues [6]. Focusing on diabetes distress is considered as a global approach to psychological problems associated with diabetes [7]. According to the Canadian Diabetes Association, diabetes distress is defined as “the despondency and emotional turmoil related specifically to having the condition, the need for continual monitoring and treatment, persistent concerns about complications and the potential erosion of personal and professional relationships” [8].
The prevalence of severe diabetes distress varies from 10 – 30% across settings and countries [9]. A recent study showed that the prevalence reached 39% in Canadian type 2 diabetics [10]. Diabetes distress is significantly associated with self-care behavior and poor glycemic control, possibly due to stress hormones leading to hyperglycemia [9, 10]. Therefore, diabetes distress has been recommended for routine screening by the American Diabetes Association and Canadian Diabetes Association [8,12]. There are two instruments accepted as measurements of diabetes distress: The Problem Areas in Diabetes scale (PAID) and the Diabetes Distress Scale (DDS) [13].
Diabetes Distress Scale was developed by Polonsky et al. in 2005 to measure four central domains of diabetes distress: emotional burden, physician care, disease management and interpersonal support [14]. The instrument surmounted limitations of several previous instruments such as the ATT39, the Questionnaire on Stress in Patients with Diabetes-Revised (QSD-R) and also the Problem Areas in Diabetes Scale (PAID) [14]. Instruction to apply DDS in clinical practice was officially introduced by the Canadian Diabetes Association in 2016 [15]. An overall average score of less than 2.0 is considered as “little or no distress”, 2.0 – 2.9 is interpreted as “moderate distress”, from 3.0 and above indicates “high distress” [16], DDS has been translated and culturally adapted to different languages worldwide [17-24] but not available in Vietnamese.
There was no reliable and valid instrument to assess distress levels in diabetics that could lead to unknown prevalence of distress in diabetes and consequently underestimate the effect of psychological issues on diabetes self-management and glycemic control in Vietnam. This study was to translate and culturally adapt the Diabetes Distress Scale into Vietnamese and evaluate its internal consistency, face and content validity.
2. METHOD
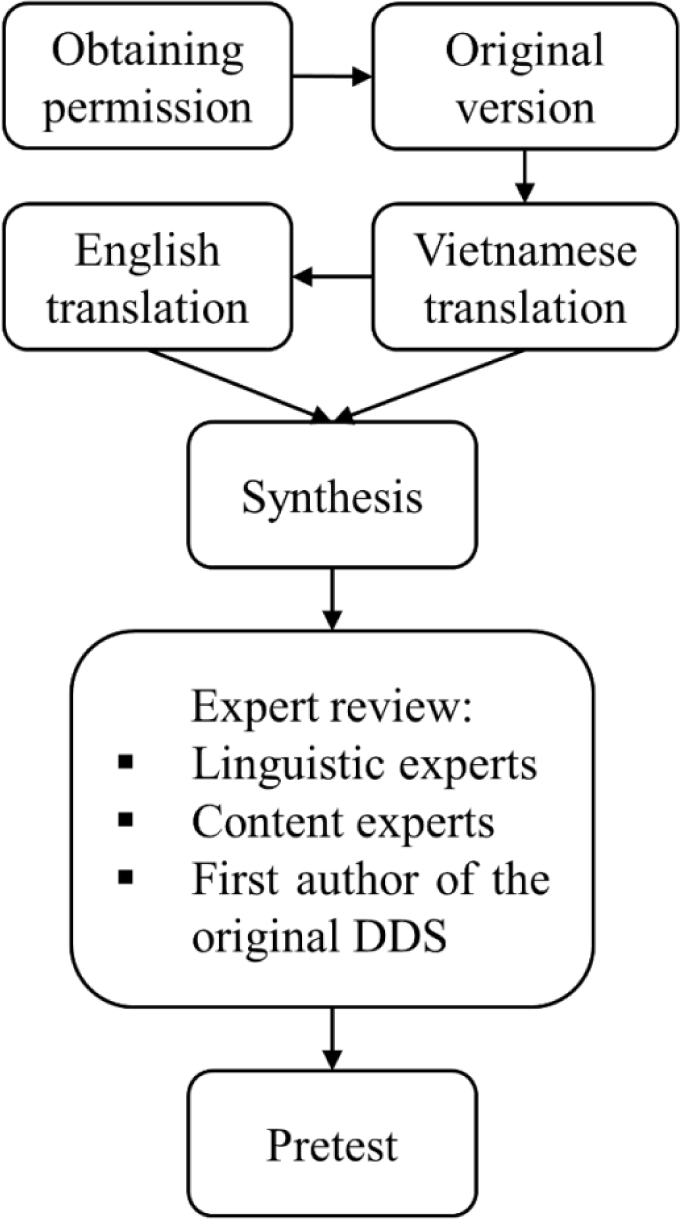
The translation process was based on standard guidelines from Guillemin et al. [24] for cross-cultural adaptation of health-related quality of life measures. The procedure included five steps: forward translation, back translation, synthesis, expert committee review and pretesting.
After permission to use and translate the DDS was obtained, forward translation of the DDS was performed by two independent translators: the former was a member of the study group who was aware of the concepts and objectives of the instrument and the latter was a qualified English translator who was not informed about these details.
Each forward translation was back-translated from Vietnamese to English independently. This stage was carried out by two translators who were bilingual overseas Vietnamese in the United States and Australia without prior knowledge of the original instrument. Discussion between four translators was held to combine all translated version into a synthesis. Any issue was resolved by repeated online discussion until a consensus was reached.
The instrument was first evaluated by a committee of linguistic experts consisting of three qualified English language specialists to access the semantic and idiomatic equivalence between the source and the target version. Then, a panel of six content experts specializing in multidisciplinary areas relevant to the study evaluated the experiential and conceptual equivalence. The expert panel consisted of two family doctors, two endocrinologists, a psychologist and a specialist in instrument development. The number of experts in each committee followed guidelines by Lynn et al [25]. Linguistic and content experts were asked to rate 17 items for their relevance and clarity compared with the original instrument using a four-point Likert content validity scale. A cover letter with instructions was included to guide experts for scoring method.
Experts were also invited to suggest modifications to improve the quality of the instrument. Any suggestion was reported and applied based on majority rule, then synthesized into a prefinal version by another member of the study group. This version was translated back to English by another bilingual overseas Vietnamese in the United States who was not aware of the concept explored and then was reviewed by the first developer of the original DDS to ensure equivalence with the original instrument.
The pretest was conducted on a sample of 31 type 2 diabetics in the Endocrinology outpatient clinic at Trung Vuong Hospital. Inclusion criteria were adults who were diagnosed with type 2 diabetes for at least 1 year and visited the clinic for routine follow-up. Patients who were pregnant or had psychiatric/psychological disorders that caused cognitive impairments could not complete the interviews were excluded from the study. In additions, patients with severe health problems such as life-threatening diseases, recent acute complications or injuries were also excluded.
All participants were asked to evaluate difficulty of understanding the items by interview after they finished taking the DDS with the following questions: “How do you feel about the questionnaire?” and “Is there any difficulty in understanding the questionnaire?”. Regardless of the answer, participants were asked to suggest modifications to improve clarity and appropriateness of each item with regards to their cultural background, which was the face validity of lay people [26].
Data entry was performed by Epidata 3.1 and statistical analysis was conducted using Stata 14.0.
Frequencies and percentages were used to describe categorical variables, medians and interquartile range were used to describe quantitative variables. Content validity was determined based on experts concurrence using content validity index for items (I-CVI). I-CVI was computed as the number of experts giving rating 3 or 4 to an item divided by the total number of experts. Based on guidelines by Lynn et al. [25], when there are five or fewer experts, a consensus of all experts must be obtained to justify the item is content-valid and equivalent with an I-CVI of 1.00. When there are more than five members in the expert panel, I-CVI must reach a minimum of 0.83, which means a disagreement is allowed.
To adjust for chance agreement, multi-rater kappa statistic was calculated [27]. First, the probability of chance agreement was obtained by the following formula:
Pc: probability of chance agreement
N: number of experts in a panel
A: number of experts rated 3or4toan item
Kappa was computed using the following formula:
Kappa was interpreted using the following criteria: 0.40 – 0.59 = fair, 0.60 – 0.74 = good, > 0.74 = excellent [27].
Internal consistency was measured using Cronbach’s alpha. Values from 0.70 to 0.95 are considered as acceptable range of Cronbach’s alpha [28].
3. RESULTS
All linguistic experts agreed that the translation correctly and fully rendered the meaning of words from the original instrument. I-CVI value was 1.00, which was interpreted as excellent for 17 items. The instrument was then evaluated by content experts and received I-CVI value from 0.83 – 1.00, corresponding to kappa value range from 0.82 – 1.00, which was also interpreted as excellent equivalence and clarity.
Most content expert committee members agreed to include the subject “tôi” and remove the word rằng” to improve the clarity of the instrument. The term “tiểu đuờng” was replaced by “đái tháo đuờng” as it is a more formal translation of “diabetes” nationwide in Vietnam. Items 1, 3, 4, 5, 8, 9, 10, 14, 15, 16, 17 archived a concordance rate of 100% among expert committee members and were therefore not subject to any modifications. Other items went through some simple grammatical changes or word replacement for corresponding synonymous. The terms “không am hiểu lắm” (item 2), “cảm thấy” (item 6), “sẽ biến chứng” (item 7), “không quan tâm lắm” (item 11), “chua tuân thủ lắm” (item 12), “khó khân đến nhuờng nào” (item 13) were replaced by “chua hiểu đầy đủ”, “nhận thấy”, “sẽ có nhũng biến chứng”, “không quan tâm đầy đủ”, “chua tuân thủ chặt chẽ”, “su khó khăn khi phải sông chung với bệnh đái tháo đuờng”, respectively, to ensure comprehension of the population.
After modification of terminology, the prefinal version was obtained, back-translated, reviewed by the first developer of the original intrument and pretested.
A total of 31 patients agreed to participate in pretesting. There were 25 patients (81.6%) answered that the items were “easy to understand” and 6 patients (19.4%) answered that the items were “very easy to understand”. All patients stated that no modification was needed for 17 items, and that they were able to complete the instrument within 15 minutes.
Participant sociodemographic characteristics and clinical data are described in Table 5. Participants were aged between 41 and 72 years, with diabetes duration range from 1 to 24 years.
The overall Cronbach’s alpha for the Vietnamese version of DDS was 0.94. Cronbach’s alpha was lowest in “Physician distress” subscale (0.76) and highest in “Interpersonal distress” subscale (0.93). All 17 items had corrected item-total correlationvalue of greater than 0.40.
4. DISCUSSION
The translation guidelines by Guillemin et al. [24] applied in this study were also used by Chew et al. [20] to translate the Malaysian version of DDS. Nevertheless, due to the lack of consensus and evidence in existing cross-cultural adaptation methods [29], any step which was not strictly instructed in the mentioned guidelines were modified with credible reasons based on other guidelines’ suggestions. The essential role of the back translation step is controversial [29], Therefore, in this study, back translations were used only as references to suggest the better between two forward translated version, not the determining factor to make a decision. Crucial decisions were made base on evaluation and consensus among committees rather than opinion of any individual [30], Membership in the committees were organized with an aim of increasing the number and credibility of experts in later stages of review. This technique was expected to ensure any bias remaining from the previous stages would be revised and corrected in the later stages by more experienced experts. Moreover, back translation of the prefinal version was submitted to the first developer of the original instrument to review for its equivalent with the English version, as proposed by Beaton et al. [30] The face and content validity were checked at the same time as the adaptation with focus group and committees using qualitative and quantitative method, respectively, as proposed in literature [29].
Except minor changes in grammar and word replacements, no significant modification required to fit the context of Vietnamese culture. This was similar to most other translated versions of the DDS in Asian population such as Hong Kong [17], Malaysia [20], Indonesia [31]. In Thailand, the term “doctof’ was replaced by “doctor or nurse” because of the prominent role of nurses in the inpatient healthcare service [19], but this was not the case as this study was conducted at the secondary hospital where patients prefer spending most time with doctors. The Vietnamese version of DDS met equivalence criteria to the original instrument because it was evaluated by the researchers, experts, first author of the original instrument and the patients. Most items received an agreement of 100% in the analysis performed by the rating of committee ofjudges and patients, which resulted in excellent content and face validity.
The Vietnamese version of DDS showed good internal consistency as all subscales had Cronbach’s alpha greater than 0.70. Cronbach’s alpha was lowest in “Physician distress” subscale (0.76), especially reflected in the item-total correlation value (0.50) of item 11 “Feeling that my doctor doesn’t take my concerns seriously enough”. It could be explained by the hospital overload in Vietnam that reduced time length of physical examination and consultation. Therefore, doctors did not have enough time to take more concerns of their patients, while their priorities were to provide knowledge (item 2) and instructions on how to manage diabetes (item 5). In this study, “Physician distress” subscale had the lowest Cronbach’s alpha compared to other subscales, but its value was similar to Brazillian version (0.77) of DDS [22]. Overall, Cronbach’s alpha of the Vietnamese version was similar to previous studies [17-21], especially the original DDS [14], demonstrated good reliability.
This study was the first to systematically translate and culturally adapt the DDS into Vietnamese language, allow other studies to evaluate diabetes distress in Vietnamese outpatients. However, further studies is needed to assess other psychometric properties of this instrument, especially the agreement between the brief instrument DDS2 developed by Fisher et al. [32] with 2 items for screening diabetes distress in clinical practice as proposed by the Canadian Diabetes Association [15] and this full DDS version with 17 items in Vietnamese culture.
There are some limitations in this study. Due to convenience sampling, the percentages of participants with high school and further education graduation added up to 48.4%. Highly educated patients might be more likely to agree to participate in the study and tend to answer that the questionnaire was easy to understand with regards to their educational background. The sample size was small and restricted to only those who were diagnosed with type 2 diabetes for at least one year to ensure all participants were fully experienced with diabetes treatment, healthcare services and self-management. These established conditions are important when assessing the patients perceptions of the instrument but they just served the purpose of evaluating the face validity of the DDS. Therefore, future studies with larger sample size and greater diversity are needed to prove other psychometric properties of the DDS.
5. CONCLUSION
In conclusion, the Vietnamese version of DDS found satisfactory results as regards the translation and cultural adaptation process. This instrument is equivalent to the original English version, reliable, face-valid, content-valid, and ready for collecting data and assessing diabetes distress among individuals with type 2 diabetes in Vietnam.