1. INTRODUCTION
The SARS-CoV-2 virus is an infectious agent that has caused a pandemic of acute respiratory infections worldwide in 2020. Many new strains of SARS-CoV-2 have appeared in later times [1]. In general, coronavirus disease 2019 (COVID-19) test results are often used to support diagnosis and monitor the effectiveness of the treatment regiments. In addition, the treatment regimens for COVID-19 patients have evolved as the disease progressing. Many previous studies have evaluated the changes in biochemical and hematological parameters related to disease severity and mortality in COVID-19 patients. Several studies compared biological and hematological parameters in mild, moderate, and severe disease groups [2]–[4], [5]. Other studies compared the differences in test indicators between survivor and non-survivor patients and developed a predictive model of COVID-19 patient mortality risk [6],[7]–[11]. Hematological parameters thought to be associated with the severity of COVID-19 infection include white blood cell (WBC), %NEU, %LYM, and monocytes (MONO), while biochemical parameters associated with COVID-19 include LDH, fibrinogen, C-reactive protein (CRP), ferritin, prothrombin, and random blood glucose (RBS). However, the results of previous studies have not been consistent [2]–[11]. In Vietnam, only a few studies have been conducted on the changes in biochemical and hematological parameters of COVID-19 patients. This study aimed to evaluate the relationship between biochemical and hematological parameters and disease severity in COVID-19 patients.
2. METHODS
The clinical characteristics, hematological parameters, and biochemical parameters of COVID-19 patients who aged 18 years old or older were investigated in this retrospective and cross-sectional study.
This study collected information on patients infected with COVID-19 who were treated at the intensive care unit (ICU) Department and the Department of Moderate COVID-19 Patients at Dong Nai General Hospital from August to December 2021.
Medical information from COVID-19 patients was collected through HIS-FP software and handwritten medical records, including age, sex, weight, height, laboratory parameters, and clinical status. There were two patient groups classified according to the disease severity at admission or treatment outcome (survivor and non-survivor groups) based on the clinician’s assessments recorded in the medical records of COVID-19 patients. Blood samples were collected from COVID-19 patients upon admission to Dong Nai General Hospital for biochemical and hematological tests. Changes in biochemical and hematological parameters in patients were determined based on the normal range of laboratory values. All biochemical and hematological parameters between the two patient groups were compared. Factors affecting treatment outcomes in COVID-19 patients were evaluated.
All clinical characteristics, hematological parameters, and biochemical parameters from COVID-19 patients aged 18 years old or older were collected at the ICU Department and the Department of Moderate COVID-19 Patients at Dong Nai General Hospital from August to December 2021. The real time RT-PCR technique was used on the Light Cycler 96 and Cobas Z480 systems to diagnose COVID-19 patients at Dong Nai General Hospital. The result was positive for SARS-CoV-2 when the cycle threshold (CT) value was less than or equal to 37. The result was negative for COVID-19 with a CT value >37. Exclusion criteria included patients whose records did not include biochemical and hematological test results; patients who were pregnant; patients who died outside hospital; patients with mild COVID-19 infection; and patients who were transferred to another hospital.
The independent variables included biochemical parameters and hematological parameters from COVID-19 patients. The dependent variable was the treatment outcome (survivors and non-survivors).
The hematological parameters of COVID-19 patients were determined on the AMP Accos 5110 hematology analyzer (AMEDA Labordiagnostik). Normal values of hematological parameters included total WBC (4.8–10.8 K/µL), NEU (1.4–6.5 K/µL), LYM (1.2–3.4 K/µL), MONO (0.1–0.6 K/µL), red blood cells (RBC; 4.2–6.1 M/µL), hemoglobin (HGB; 12–18 g/dL), PLT (130–400 K/µL), prothrombin time (PT; 70%–140%), activated partial thromboplastin time (aPTT; 25.4–36.9 s), %NEU (42%–75.2%), %LYM (20%–51.1%), MONO% (1.7–9.3), neutrophil/lymphocyte ratio (NLR)<3, platelet/lymphocyte ratio (PLR; 50–150), red cell distribution width (%RDW; 11.5%–14.5%), and international normalized ratio (INR; 0–1.2).
Biochemical parameters of COVID-19 patients were determined on AU680 clinical chemistry analyzer (Beckman-Coulter). Normal values of biochemical parameters included RBS (3.4–11 mmol/L), CRP≤6 mg/L, ferritin (21.81–274.66 ng/mL), D-dimer≤500 ng/mL, procalcitonin≤0.046 ng/mL, AST≤37 U/L, ALT≤40 U/L, LDH (135–225 U/L), creatinine (53–120 µmol/L), urea (2.5–7.5 mmol/L), and eGFR≥60 mL/min/1.73 m2. Body mass index (BMI) was calculated and categorized according to the Asian-Pacific cutoff points [12].
The questionnaires and sampling/exclusion criteria were designed for this study to collect patients’ information that was relevant to the study and to avoid factors that may distort the results of the study, such as wrong study subjects or errors in data collection.
The number of patients in the study (N) was determined according to the formula N=(Z1–α/2)2×p(1–p)/d2, where p was the prevalence of COVID-19 patients in Vietnam as 12% [13]; d was the acceptable error range, not more than 5%; Z was the coefficient of confidence (Z value is 1.96 with a 95% confidence level). According to the formula, the minimum number of patients for this study was 102. However, 654 medical records that met the sampling criteria were included in this study.
Quantitative variables, including biochemical and hematological parameters, were divided into treatment outcome groups. Results were presented as mean (mean±SD) for normally distributed continuous variables or as median (interquartile range [IQR]) for non-normally distributed continuous variables.
Software, including Microsoft Excel 2013 and IBM SPSS Statistics 29 (IBM, Chicago, IL, USA), was used to process statistical data. Mann-Whitney and Kruskal-Wallis were the statistical tests used to compare the means of two and three groups of variables, respectively, that were not normally distributed. The T-test was used to compare the mean value of the two groups of independent variables with a continuous normal distribution, while the Chi-square test (χ2) was used to compare the frequencies of categorical variables. The p-value<0.05 showed that the results were significant.
3. RESULTS
The study investigated more than seven hundred medical records of COVID-19 patients treated at the ICU Department and the Department of Moderate COVID-19 patients at Dong Nai General Hospital from August to December 2021, but only six hundred and fifty-four patients met the inclusion criteria for this study.
Among 654 COVID-19 patients , there were 173 moderate patients (26.5%), 109 severe patients (16.7%), and 372 critically illed patients (56.9%). During the treatment, 308/654 COVID-19 patients died, accounting for 47.1%. Most patients in the moderate and severe groups were discharged (97.7% and 88.1%, respectively), in contrast to the critical group with 78.2% deaths (Fig. 1).
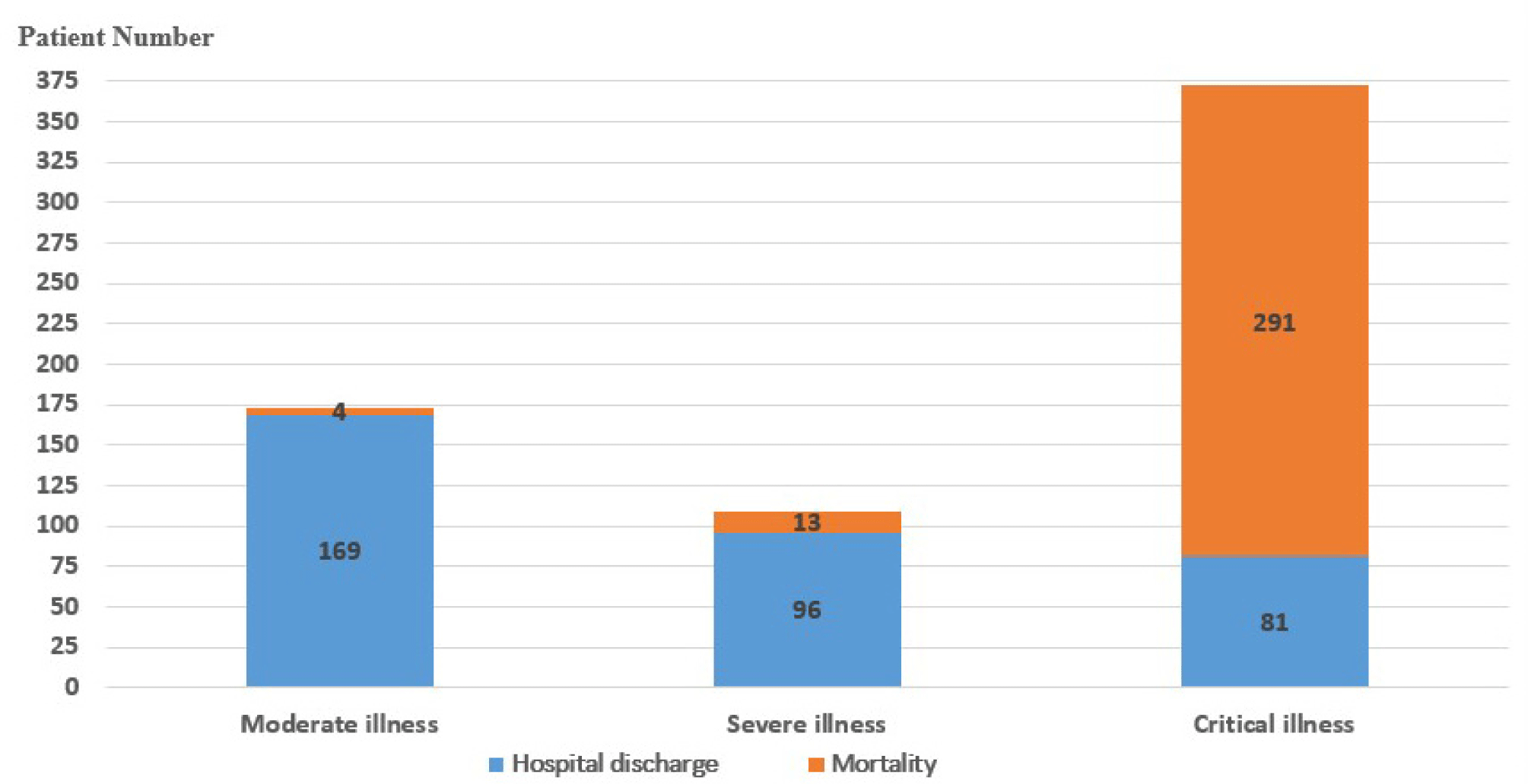
The proportion of COVID-19 female patient infected with was higher than that of men (56.9% vs. 43.1%). COVID-19 patients have an average age of 56.7±16 years old (ranging from 19 to 96 years old), of which patients over 60 years old accounted for 44%. Non-survivors had a mean age of 62.5±14.4 years old and were significantly older than the survivors (51.5±15.5 years old) (p<0.001). There were 324/654 overweight patients (35.8%), of whom the number of overweight patients who died was significantly higher than the number of overweight patients who survived (p=0.006) (Table 1).
| Characteristics |
Total (N=654) n (%) |
Discharged survivors (N=346) n (%) |
Non-survivors (N=308) n (%) |
p-value | |
|---|---|---|---|---|---|
| Sex | Male | 282 (43.1) | 150 (43.4) | 132 (42.9) | 0.898 |
| Female | 372 (56.9) | 196 (56.6) | 176 (57.1) | ||
| Age (yr) | ≤40 | 116 (17.7) | 96 (27.7) | 20 (6.5) | <0.001 |
| 41–60 | 250 (38.2) | 137 (39.6) | 113 (36.7) | ||
| ≥60 | 288 (44) | 113 (32.7) | 175 (56.8) | ||
| Mean±SD | 56.7±16 | 51.6±15.5 | 62.5±14.4 | <0.001 | |
| BMI [12] (kg/m2) | Underweight (<18.5) | 45 (6.9) | 26 (7.5) | 19 (6.2) | 0.006 |
| Normal weight (18.5–22.9) | 375 (57.3) | 209 (60.4) | 166 (53.9) | ||
| Overweight (23–24.9) | 127 (19.4) | 71 (20.5) | 56 (18.2) | ||
| Obese (≥25) | 107 (16.4) | 40 (11.6) | 67 (21.8) | ||
| Consciousness | Yes | 599 (91.6) | 340 (98.3) | 259 (84.1) | <0.001 |
| No | 55 (8.4) | 6 (1.7) | 49 (15.9) | ||
| Numbers of comorbidities | None | 226 (34.6) | 123 (35.5) | 103 (33.4) | 0.322 |
| 1 | 202 (30.8) | 116 (33.5) | 86 (27.9) | ||
| 2 | 156 (23.9) | 74 (21.4) | 82 (26.6) | ||
| 3 | 55 (8.4) | 26 (7.5) | 29 (9.4) | ||
| ≥4 | 15 (2.3) | 7 (2) | 8 (2.6) | ||
| Comorbidities | Hypertension | 202 (30.8) | 98 (28.3) | 104 (33.8) | 0.133 |
| Diabetes | 161 (24.6) | 68 (19.7) | 93 (30.2) | 0.002 | |
| GERD | 82 (12.5) | 61(17.6) | 21 (6.8) | <0.001 | |
| CKD | 42 (6.4) | 13 (3.8) | 29 (9.4) | 0.003 | |
| Respiratory diseases | 36 (5.5) | 21 (6.1) | 15 (4.9) | 0.502 | |
| Cerebrovascular complications | 31 (4.7) | 10 (2.9) | 21 (6.8) | 0.018 | |
| Coronary artery disease | 26 (4) | 8 (2.3) | 18 (5.8) | 0.021 | |
| Cancer | 19 (2.9) | 9 (2.6) | 10 (3.2) | 0.624 | |
| Liver diseases | 19 (2.9) | 12 (3.5) | 7 (2.3) | 0.364 | |
| Heart failure | 17 (2.6) | 6 (1.7) | 11 (3.6) | 0.140 | |
| Lipid disorders | 8 (1.2) | 7 (2) | 1 (0.3) | 0.072 | |
| Anemia | 8 (1.2) | 7 (2) | 1 (0.3) | 0.072 | |
| Hospital duration (day) | 12 (8–17) | 12 (9–17) | 11 (7 –16) | 0.001 | |
| SpO2 (%) | 86.5±13.9 | 91.4±9.3 | 81±15.9 | <0.001 |
The majority of COVID-19 patients had no comorbidities (34.6%). The remaining patients had 1-4 comorbidities, but the number of comorbidities between the two survivor and non-survivor groups was not significantly different (p=0.332). The most common comorbidities were hypertension with a frequency of 30.8%, followed by diabetes (24.6%), gastritis–gastroesophageal reflux disease (GERD; 12.5%), chronic kidney disease (CKD; 6.4%), and other diseases with lower rates. The proportion of patients with diabetes, CKD, cerebrovascular complications, and coronary artery disease was statistically different between the group of survived patients and the group of non-survived patients (p=0.002, p=0.003, p=0.018, and p=0.021, respectively). The average SpO2 value of COVID-19 patients was 86.5±13.9% which was below the allowable threshold (<96%). The hospital duration in the group of non-survived patients was significantly shorter than in the group of survived patients (p=0.001) (Table 1).
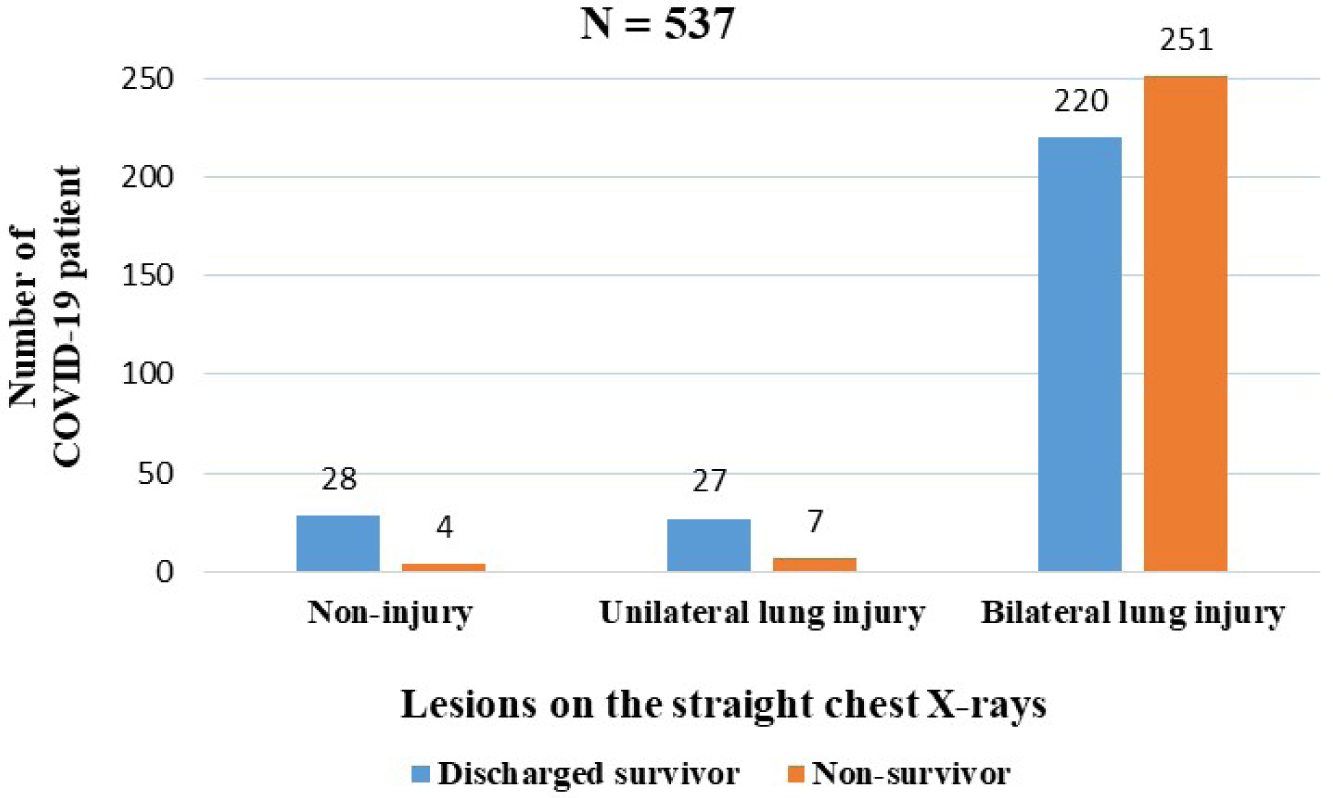
Among the 654 COVID-19 patients , only 537 patients had chest X-rays and 89 patients had their blood type determined immediately upon admission. 471 patients had bilateral lung injury (87.7%), in which the percentage of bilateral lung injury in the discharged survivors was lower than non-survivors (Fig. 2). The proportion of COVID-19 patients with blood types O and B was 37.1 and 33.7%, respectively, and the lowest was with blood group AB (11.2%). There were negligible differences in blood types between non-survivors and discharged survivors with COVID-19 (p>0.05).
There were various changes in hematological parameters in 654 COVID-19 patients in this study. Hematological parameters of COVID-19 patients were all increased compared to the reference thresholds, including WBC, NEU, and %NEU, NLR, and PLR. In particular, mean WBC counts and %NEU were increased in COVID-19 non-survivors but not in discharged survivors. The NLR of COVID-19 non-survivors was twice that of discharged survivors. In contrast, the LYM and %LYM of COVID-19 patients were lower than the reference threshold. The %LYM of COVID-19 non-survivors was twice as low as that of discharged survivors. Other hematological parameters of the patient population were within the limits of the reference threshold. There were significant differences in WBC, LYM, %LYM, NEU, %NEU, percentage of monocytes (%MONO), %RDW, NLR, and PLR between the two groups of discharged survivors and COVID-19 non-survivors (p<0.05). The mean PLT levels of COVID-19 non-survivors were not significantly lower than those of discharged survivors (p>0.05) (Table 2).
COVID-19, coronavirus disease 2019; IQR, interquartile range; WBC, white blood cells; NEU, neutrophils; LYM, lymphocytes; MONO, monocytes; %NEU, percentage of neutrophils; %LYM, percentage of lymphocyte; HGB, hemoglobin; %MONO, percentage of monocytes; RBC, red blood cells; RDW, red cell distribution width; PLT, platelets; NLR, neutrophil/lymphocyte ratio; PLR platelet/lymphocyte ratio.
Biochemical parameters of COVID-19 patients with average values higher than the reference threshold included AST, LDH, CRP, and D-dimer. LDH enzyme activity, CRP, and D-dimer levels were increased in both discharged survivors and non-survivors with COVID-19. In particular, the mean LDH activity, mean CRP concentration, and mean D-dimer concentration in non-survivors were more than twice as high as in discharged survivors, while the mean AST activity was increased only in non-survivors with COVID-19. Other biochemical parameters of the patient population were within the range of the reference threshold. Significant differences were found in RBS, urea, creatinine, procalcitonin, and D-dimer levels, AST and LDH activities, aPTT, and estimated glomerular filtration rate (eGFR) values between discharged survivors and non-survivors with COVID-19 (p<0.05) (Table 3). Since some patients with COVID-19 had ferritin levels greater than 2,000 ng/mL, the mean or median value of ferritin could not be calculated. In this study, 82.9% of COVID-19 patients had an increase in ferritin levels above the reference threshold, and no patients had a decrease in ferritin levels. The proportion of COVID-19 non-survivors with ferritin levels over 1,000 ng/mL was 3.5 times higher than discharged survivors with ferritin levels over 1,000 ng/mL (77.9% vs. 22.1%), in which COVID-19 patients with ferritin levels over 2,000 ng/mL were mainly found in the non-survivor group.
COVID-19, coronavirus disease 2019; IQR interquartile range; RBS, random blood glucose; AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; PT, prothrombin time; INR, international normalized ratio; aPTT, activated partial thromboplastin time.
4. DISCUSSION
In this study, we analyzed data from the medical records of 654 COVID-19 patients who were treated at the ICU and COVID-19 patient departments of Dong Nai General Hospital from August to December 2021. Because Dong Nai General Hospital was the unit in charge of collecting and treating patients with moderate to severe COVID-19 disease, the proportion of severe to critical patients was quite high (accounting for 56.9%). There were 308 deaths found in a total of 654 patients, accounting for 47.1%. This rate was slightly higher than the mortality rate in the study of Qiu et al. (37.6%) [14], and higher than in the study of Gray et al. (26.6%) [15]. The cause of the high mortality rate may be due to the low rate of vaccination against COVID-19 at that time. Moreover, many COVID-19 patients were elderly and had comorbidities such as diabetes and high blood pressure. In this study, the proportion of COVID-19 female patients accounted for 56.9% of the population , lower than the proportion of female patients in the study of Khalid et al. (68%) [6]. However, other studies have reported contrast results. Ghazanfari et al. reported 77.4% of men infected with COVID-19 [16]. The study by Huyut et al. concluded that male gender was a factor associated with mortality from COVID-19 infection [10]. Differences in the gender ratio of COVID-19 patients in these studies may be due to ethnic, geographical, or other factors. In terms of age, the population in this study had an average age of 56.7±16 years old, of which patients over 60 years old accounted for the highest proportion (44%). The highest mortality rate was found in patients over 60 years of age. Thus, older age has an impact on mortality in COVID-19 patients. Bilgir et al. reported that the mean age of patients with COVID-19 was 56±18.5 years old [5]. Elderly patients have a greater risk of disease progression and a less favorable prognosis, which can be explained by declining resistance, increasing age, and underlying diseases. Most of the patients with COVID-19 in this study had comorbidities (66.4%) and could have a double risk of death. The most common comorbidities were hypertension (30.8% of patients) and diabetes (24.6%). This result was similar to Kantri et al.’s study, but with a lower prevalence (26.9% hypertension and 14.2% diabetes) [3]. This study found a difference in BMI between survivors and non-survivors with COVID-19 (p=0.006). Kwok et al. suggested that obesity is a factor related to COVID-19 infection [17]. In addition, the percentage of COVID-19 patients with blood group O was the highest, similar to the study by Hwaiz et al. [18]. However, Rahman et al. showed that the risk of COVID-19 infection was lower in people with blood type O [19].
Regarding hematological parameters, the average WBC was significantly higher in non-survivors with COVID-19 than in the discharged survivors (p<0.001). This result was similar to another study on Vietnamese patients in Hue City by Nguyen et al. [20]. Other studies reported that WBC elevation affected severity and mortality in COVID-19 patients [3],[9],[10]. The number of NEU and %NEU increased while the number of LYM and %LYM decreased compared to the reference threshold. This finding is consistent with some studies in critically illed patients [4],[8],[21]. Nguyen et al. found an increase in %NEU and a decrease in %LYM in Vietnamese COVID-19 patients, as well as a significant difference in these parameters between the death and discharge groups [20]. The ratios of NLR and PLR are commonly used as predictors of COVID-19 mortality [22],[23]. The NLR and PLR found in this study were significantly lower in the discharge group than in the death group (p<0.05), similar to the study of Ghosh et al. [8]. A cut-off value of NLR>5 was associated with a high risk of sepsis [20]. Additionally, LYM depletion increased PLR. Therefore, PLR can be considered a mortality predictor in patients with severe COVID-19 infection [22]. We found a statistical difference in RDW between the discharged group and the death group. According to Bilgir et al., RDW is important for predicting mortality with higher sensitivity and specificity than hemoglobin [5]. Regarding the change in biochemical parameters of COVID-19 patients in this study, 83.1% of patients had CRP levels above the reference threshold, in which the death group had higher CRP concentrations than the discharged group (p<0.001). Some studies have shown that CRP levels are closely related to disease severity [22], but other research has concluded that CRP levels are not helpful for predicting the severity of COVID-19 [24]. The proportion of patients with ferritin concentrations over 1,000 ng/mL in the death group was higher than in the discharged group (77.9% vs. 36.2%; p<0.001). Cheng et al. discovered that ferritin concentrations were higher in the death group than in the discharged group [25]. Furthermore, patients with one or more comorbidities, such as diabetes, clotting disorders, or cancer, had higher ferritin levels than those without (p=0.01) [25]. The proportion of COVID-19 patients in this study with procalcitonin levels above the threshold was 92.3%. Procalcitonin levels increased significantly in the death group compared with the discharged group (p<0.001). Huyut et al. showed that procalcitonin levels were significantly increased in the death group compared to the survival group and confirmed that procalcitonin increased the risk of death in COVID-19 patients [10]. Regarding plasma enzyme tests, the proportion of COVID-19 patients in this study with elevated enzyme activity of AST, ALT, and LDH would be 61.4%, 46.7%, and 80.5%, respectively. However, only AST and LDH were significantly associated with treatment outcome (p<0.001). The incidence of liver disease in COVID-19 patients in this study was 2.9% and was higher in discharged survivors than in non-survivors. Therefore, AST may be elevated due to liver disease. However, previous studies have concluded that the increase in AST and ALT levels could be due to the damaged hepatocytes being destroyed during the cytokine storm [14],[26]. LDH abnormalities in COVID-19 patients were found to be affected by severe and critical disease conditions. LDH activities were higher in the death group than in the discharged group (p<0.001). Qiu et al. also concluded that increased LDH is associated with an increase in mortality in COVID-19 patients [14]. D-dimer has been considered a biomarker in cases of coagulopathy with COVID-19. In this study, D-dimer levels were significantly higher in the death group than in the discharged group (p<0.001). Age over 60 and CKD are factors that increase D-dimer levels in COVID-19 patients. Khalid et al. discovered that COVID-19 patients had higher D-dimer levels than healthy subjects [6]. Zhou et al. also found that D-dimer levels in the death group were significantly higher than in the discharged group [11]. According to Bilgir et al., D-dimer is one of the best predictors of mortality risk [5]. Furthermore, parameters related to renal function such as urea, creatinine, and eGFR were found to be significantly different between the death group and the discharged group (p<0.001). The incidence of CKD in this study was higher in the death group than in the discharged group with COVID-19. Therefore, there may be a relationship between CKD and other biochemical parameters in COVID-19 patients.
Currently, studies on changes in biochemical and hematological parameters in COVID-19 patients in Vietnam are limited. The COVID-19 epidemic evolves over time due to many new strains appearing with different characteristics. Our study is the first on COVID-19 patients at Dong Nai General Hospital. Therefore, our study cannot be extrapolated to all COVID-19 patients in other regions of Vietnam. Moreover, the limitation of this study was that it only investigated biochemical and hematological parameters in COVID-19 patients without assessing the factors affecting the change of these parameters, such as disease severity, comorbidities, and use of drugs. Therefore, assessment of factors affecting the change of biochemical and hematological parameters in COVID-19 patients should continue further in future studies.
5. CONCLUSION
Increased hematological parameters included WBC, NEU, %NEU, NLR, and PLR, while LYM and %LYM were significantly decreased in non-survivors compared with survivors. Biological parameters, including AST, LDH, CRP, and D-dimer, were significantly increased in non-survivors in comparison to survivors. Therefore, hematological and biochemical parameters should be considered to assess the severity and risk of mortality in COVID-19 patients.
